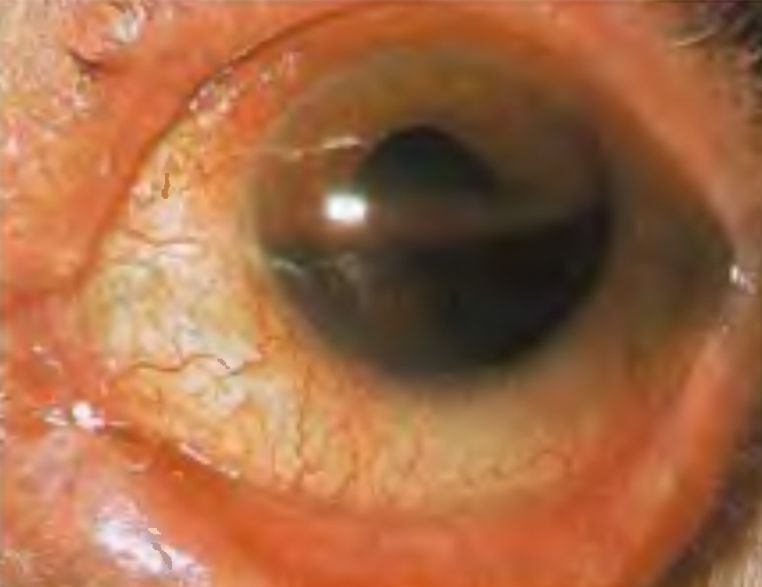
The Case
A 25 year old man presents 5 hours after an injury sustained in an intense racquetball match. He states that he had not been wearing protective eyewear at the time and that a ball hit him directly in his left eye. He reports occular pain and immediate blurry vision that has progressively worsened since his injury. A quick pen light examination of the left eye reveals an obvious hyphema filling just over a third of the anterior chamber.
[bg_faq_start]What is a hyphema?
Hyphema refers to the presence of blood in the anterior chamber of the eye. A microhyphema is a hyphema visible only with the aid of a slit lamp examination. In the examination of a patient with ocular trauma, a hyphema is an injury that can portend both acute and long term sequelae as well as being associated with other devastating ocular injuries that must be addressed in a timely manner.
The most common cause of a hyphema is trauma, usually blunt, wherein vessels of the iris and ciliary body are torn and blood enters the anterior chamber [1]. A paper studying the epidemiology of hyphema found the median age of patients to be 23 years old, with 88% of patients being male. The largest single cause of injury was sports or recreation with the majority of injuries being preventable with proper eyewear [2]. Hyphemas can also occur spontaneously without trauma as a result of a variety of disease processes. Selected causes include leukemia/lymphoma, ocular neoplasms, neovascularization of the iris, herpetic keratouveitis, complications with artificial lens implants and post-surgical hyphemas [3]. Coagulapathies and the use of anticoagulant medications are often provoking factors for other underlying causes but rarely cause hyphemas by themselves [4].
Approach and Considerations
A thorough history in a patient with ocular trauma should pay careful attention to the mechanism of injury, detailing the time, force, direction and potential for intraocular foreign bodies. Patients with hyphema commonly experience ocular pain and decreased visual acuity. The extent of visual loss and its time course should be elicited from the patient. Delayed or evolving loss of vision following the initial injury should raise suspicion for rebleeding or raised intraocular pressure [3]. A past medical history of sickle cell disorder, recent ocular surgery and the use of anticoagulant medications should be sought.
In the setting of trauma to the eye, care should be taken to consider other potential injuries including:
- Open globe injury
- Traumatic iritis
- Lens dislocation
- Corneal abrasion
- Retinal detachment
- Vitreous hemorrhage
- Orbital floor blow out fracture
A good history and physical examination can help to rule out some of these concomitant injuries. Traumatic iritis, presents within a few days of a blunt ocular injury with photophobia, redness, and ocular pain not completely relieved by topical tetracaine drops. Slit lamp examination typically reveals perilimbial conjunctival injection or “ciliary flush” and cells and flare in the anterior chamber. Diplopia may be present with either a lens dislocation or extraocular muscle entrapment from an orbital floor fracture. Diplopia from entrapment of one of the ocular muscles results in a binocular diplopia, or double vision, that resolves when one of the eyes is closed. Lens dislocation would result in monocular diplopia. Other signs of an orbital floor or “blow out” fracture includes enopthalamos, limitation of upward gaze, and sensory loss over the cheek and upper lip. Retinal detachments may also occur following blunt trauma and presents with classic signs of flashes of light, floaters and visual field cuts such as the “gray curtain” effect. Open globe injuries can be obvious or occult. Siedel’s sign is useful in the identification of an open globe injury:
Physical examination specific to hyphemas in the emergency department should include visual acuity assessment, measurement of intraocular pressure (once an open globe injury has been ruled out) and a slit-lamp examination to measure the extent of the bleed and for the presence of clotted, dehemoglobinzed blood.
Hyphemas can be graded on the following scale:
| Grade | Description |
|---|---|
| Microhyphema | Visible only by use of a slit lamp, often only circulating red blood cells floating in the anterior chamber are seen |
| Grade 1 | Less than one third of the anterior chamber |
| Grade 2 | Between one third and one half of the anterior chamber |
| Grade 3 | Greater than half of the anterior chamber |
| Grade 4 | “8 Ball” hyphema with the entirety of the anterior chamber filled with clotted blood. Hyphemas that fill the entire anterior chamber with fresh blood may become smaller once clotting occurs |
This grading system has prognostic implications and can help determine the decision for admission [5].
Consideration of screening for sickle cell disease should also be made in the appropriate patient population. Patients with sickle cell disease are at increased risk for secondary glaucoma by occlusion of the trabecular meshwork by sickled red blood cells, blocking the regular drainage of aqueous humor from the anterior chamber and leading to a rise in intraocular pressure. The use of carbonic anhydrase inhibitors such as acetazolamide is contraindicated in these patients as they may promote sickling of red blood cells by lowering the pH in the anterior chamber [6].
Management and Disposition
The emergency department management of hyphema centers on control of any ongoing bleeding within the eye, prevention of any subsequent bleeding and the anticipation and treatment of hyphema complications. A number of treatment modalities for the treatment of hyphema have been traditionally described, however there is often little evidence that these measures improve final visual acuity [7].
Use of an eye shield is recommended for prevention of any potential further trauma to eye. Elevation of the head of the bed to 35-40 degrees helps to promote settling of blood inferiorly and prevent occlusion of the trabecular meshwork by red blood cells [3]. In the past, patients were admitted and put on bed rest to minimize the risk of rebleeding, however there is significant controversy regarding the benefits of this intervention [7],. Most patients are now counseled to refrain from strenuous activity and managed as outpatients, with frequent follow-up examinations.
Topical anti-fibrinolytic drugs including aminocaproic and transexamic acid have been used in hyphemas and help to reduce the occurrence of rebleeding[8]. However there is no definite evidence suggesting they help to improve final visual acuity[7] and these are now rarely used.
A cycloplegic, such as atropine 1%, is often used with the intent of preventing pupillary movement thus limiting further movement of torn iris vessels and promoting tamponade [6]. The agent of choice is generally best directed by the consultant ophthalmologist, as certain eye drops such as atropine have a long duration of action.
Pain management is best accomplished by acetaminophen. The use of platelet inhibiting drugs is not recommended.
Recognition and management of raised intraocular pressure is of paramount importance as bleeding may block the trabecular meshwork of the eye and prevent the normal drainage of aqueous humor leading to secondary glaucoma. The normal intraocular pressure (IOP) of the eye is between 10 and 20 mmHg of mercury. Elevations above this range, especially above 35mmhg, are concerning and can lead to damage to the optic nerve. Treatment of raised IOP is usually accomplished initially with the use of a topical β-blocker such as 0.5% timolol, which acts to decrease the production of aqueous humour. Topical α2-agonist therapy with an agent such as brimonidine can also be used. Acetazolamide, a carbonic anhydrase inhibitor, also decreases production of aqueous humor at a dose of 500mg IV or PO. Hyperosmolar therapy with mannitol reduces total volume of aqueous humour through the generation of an osmotic gradient, drawing fluid into the intravascular space. Dosing is at 1 to 2 g/kg IV [3].
All patients with hyphema deserve prompt consultation by an ophthalmologist, as some complications may not be apparent until several days after the injury. Rebleeding commonly presents within the first few days following the initial injury as initial clotting retracts [3], and is associated with a worse prognosis. A hyphema may also lead to the development of membranous anterior or posterior synechiae secondary to the inflammatory process in the eye, whereby the iris may become adherent to the cornea or the lens respectively. Corneal staining by blood and angle recession glaucoma are other potential complications.
References
- Rosen’s Emergency Medicine: Concepts and Clinical Practice (8thed) 2014. Philadelphia. Elsevier Saunders Inc. – Chapter 71
- Khan-Farooqi, H. R., Chiranand, P, Edelstein, S.L. (2010). Epidemiology and Outcome of Traumatic Hyphema: A Retrospective Case Series. Investigative Opthalmology and Visual Science, 51(13), 1314.
- The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease (6th ed) 2012. Ovid Technologies, Inc. – Chapter 3
- Bagnis, A., Lai, S., Lester, M., Bacino, L., Traverso, C. E. (2008). Spontaneous Hyphema in a Patient on Wafarin Treatment. Br J Clin Pharmacol. 66(3), 414-415.
- Shammas, H.F., Matta, C.S. (1975). Outcome of Traumatic Hyphema. Ann Ophthalmol. 7(5), 701-706.
- Tintinalli’s Emergency Medicine: A Comprehensive Study Guide (7thed) 2011. New York. McGraw Hill Companies Inc. – Chapter 268
- Gharaibeh A, Savage HI, Scherer RW, Goldberg MF, Lindsley K. Medical interventions for traumatic hyphema. Cochrane Database of Systematic Reviews 2013, Issue 12. Art. No.: CD005431. DOI: 10.1002/14651858.CD005431.pub3
- Crouch, E.R. Jr, Williams, P.B., Gray, M.K., Crouch, E.R., Chames, M. (1997) Topical Aminocaproic Acid in the Treatment of Traumatic Hyphema. Arch Ophthalmol. 115(9), 1106-1112.