All the content from the Blood & Clots series can be found here.
CanMEDS Roles addressed: Medical Expert
Case Description
A 32-year old woman presents to the ED following a motor vehicle collision with lateral impact (T-bone collision) and is brought into the trauma bay. Significant bleeding was reported on scene and the patient arrives looking diaphoretic, pale, and unwell.
Vitals in the trauma bay are BP 104/64 mmHg, HR 128, RR 22 95% RA, and T 36.1. She has no known drug allergies according to the medical chart.
You conduct a rapid assessment of the patient to ensure the airway is patent and lungs clear. She responds in short sentences due to pain and dyspnea. Several facial lacerations and a bleeding right forehead laceration are present. She has significant bruising and a firm, distended abdomen. FAST ultrasound reveals a significant amount of free fluid in the abdomen
In addition to bleeding from the forehead laceration you are concerned about internal bleeding from blunt abdominal and pelvic injuries. Bloodwork including a group and screen (and crossmatch) has been sent STAT but is still pending.
Will this patient benefit from massive transfusion protocol (MTP) and when is the best time to activate it?
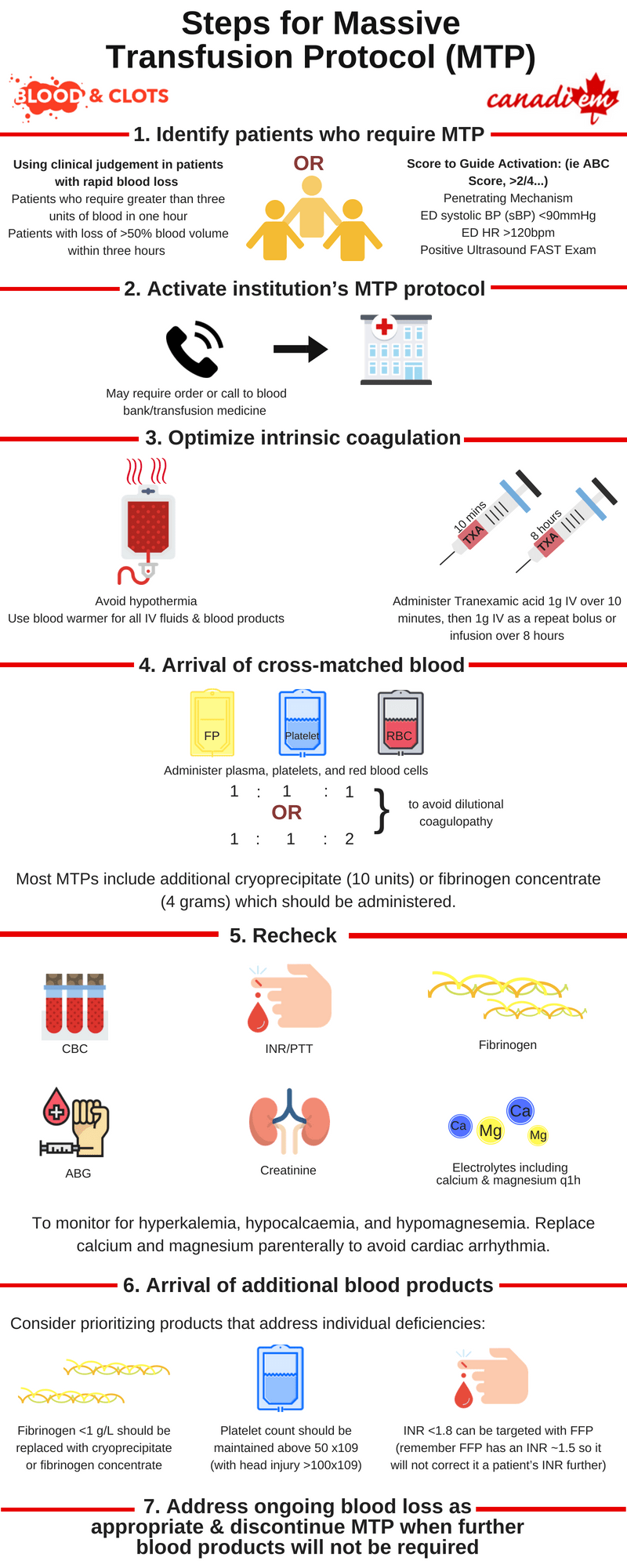
Hemorrhage is a major cause of morbidity in trauma. It is responsible for almost 50% of deaths occurring within 24 hours of injury and up to 80% of intraoperative trauma mortalities 1. In the past a commonly used definition for massive transfusion protocols (MTPs) was >10 units RBCs in 24 hours, but this is more useful for research purposes than for frontline physicians. Instead, consider activating MTPs for:
- Patients who require greater than three units of blood in one hour (or anticipated) – this is known as the critical administration threshold 2
- Patients with loss of >50% blood volume within three hours
- If ED HR/sBP > 1.4 (Shock Index) 4
- Those who meet criteria using a validated score. The most common is the ABC score 3. Consider activating MTP when 2/4 are present:
- Penetrating Mechanism
- ED systolic BP (sBP) <90mmHg
- ED HR >120bpm
- Positive Ultrasound FAST Exam
MTPs have several proposed benefits for patients who require large volume blood resuscitation. Approximately 30% of trauma patients have already developed clinically significant coagulopathy when they arrive in the emergency department 5. MTPs aim to deliver blood in a prompt and protocolized fashion. Resuscitation with crystalloid and PRBCs carries significant risk of dilutional coagulopathy and third-spacing of fluid including cerebral, cardiac, pulmonary, and renal edema 6. MTPs aim to avoid the negative effects of excessive crystalloid administration and minimize coagulopathy by administering RBCs, platelets, and plasma in a balanced ratio (approximating 1:1:1 or 1:1:2 per the PROPPR trial) 7. Administration of either cryoprecipitate or fibrinogen concentrate are also common in MTP.
Key Concepts in MTPs
The patient remains tachycardic with labile blood pressure despite two units of RBCs and two litres of crystalloid. You decide to activate the hospital’s massive transfusion protocol prior to the results of laboratory tests becoming available.
Physicians should familiarize themselves with how to activate massive transfusion protocol in their centre of practice so that blood products are not delayed in time-sensitive situations.
While you wait for the first cross-matched blood products to arrive you prepare for potential complications of MTP by completing the following:
- Ensure you have appropriate intravenous access including central venous access if necessary
- Bloodwork is sent including CBC, Group and Screen, INR, PTT, fibrinogen, ABG, creatinine, and electrolyte panel including calcium and magnesium. These should be repeated q1h initially to help guide the order of blood product administration and anticipate complications of massive transfusion
- Use of available measures to prevent hypothermia including a blood warmer for all IV fluids and blood components as well as bair hugger and warmed ambient room temperature
Administration of Tranexamic acid 1g IV over 10 minutes and then 1g IV as a repeat bolus or as an infusion over 8 hours. First dose of TXA should be given within the first 3 hours as per the CRASH-2 study 8. If venous access is complicated by infusion, the second dose can also be given as a bolus over ten minutes per the MATTERs study 9.
Maintaining normothermia is particularly critical in scenarios where blood loss is ongoing. Every 1-degree Celsius drop in temperature increases blood loss by 16% and mortality figures rise dramatically below 34 degrees. Hypothermia contributes to loss of platelet and coagulation factor activity. Cold blood has also been shown to induce cardiac arrhythmias particularly when infused rapidly through a central line.
The first MTP package arrives containing 6 units of RBCs, 6 adult doses of platelets, 6 units FFP, and 10 units of cryoprecipitate (note that in some centres fibrinogen concentrate will be used in place of cryoprecipitate).
Decisions regarding order of administration can be individualized by considering the following parameters as “critical levels” that require immediate replacement in ongoing hemorrhage
- Fibrinogen <1 g/L should be replaced immediately and with a target fibrinogen of >1.5-2.0 g/L
- Target platelet count >50 x109 (with head injury >100×109)
- INR <1.8 can be targeted with FFP (remember FFP has an INR ~1.5 so it will not correct it a patient’s INR further)
Administration of the first set of blood products has been completed. The patient’s blood pressure has stabilized and they are being prepared for transfer to the CT. A second package of products will be arriving soon. While you wait you check recent bloodwork for the electrolyte abnormalities that can occur in MTP including hyperkalemia, hypocalcaemia, and hypomagnesemia as the combination can induce myocardial depression and arrhythmia.
Hyperkalemia results from degradation of stored red blood cells, and increases with age of red blood cell product being transfused.
Hypocalcaemia and hypomagnesemia in MTP are the result of citrate toxicity. Citrate is the anticoagulant used in blood components and in MTP the amount may overcome the ability of the liver to metabolize it. Symptoms of hypocalcaemia including hypotension, narrow pulse pressures, tetany, and paresthesias are easy to overlook in unstable, multiply transfused patients. Low or decreasing blood levels of calcium and magnesium should be treated with IV replacement to prevent complications including arrhythmia and death.
Case Conclusion
You appropriately recognize a clinically unstable patient with signs of ongoing bleeding. Trauma patients are frequently coagulopathic on presentation, and you activate a massive transfusion protocol to replace the patient’s blood loss with products for volume resuscitation and promotion of hemostasis. By preventing hypothermia, and giving blood products in a balanced ratio, you give your patient the best chance at achieving hemostasis while awaiting definitive intervention.
The patient is transferred to CT and found to have contrast extravasation from the spleen. They are transferred to the operating room for laparotomy and splenectomy. Post-operatively the patient was transferred to ICU, and the remainder of the current round of blood products was transfused. Hematologic parameters remained stable over the next few hours and massive transfusion protocol can be safely discontinued.
Main Messages
- MTP is indicated for patients with prior or ongoing major hemorrhage. MTPs can prevent dilutional coagulopathy and the deleterious effects of large volume crystalloid administration. Fibrinogen, INR, and platelets should be targeted to levels shown to promote hemostasis.
- Crucial adjunctive measures include administration of tranexamic acid and prevention of hypothermia including the use of a blood warmer, bair hugger, and warmed ambient room temperature.
- Monitor calcium, magnesium, and potassium to prevent complications of MTP including arrhythmia and death.
Summary Infographic for Massive Transfusion Protocol
Additional Resources and Websites
American Society of Anesthesiologists. Massive Transfusion Protocol (MTP) for Hemorrhagic Shock. 2010
All the content from the Blood & Clots series can be found here.
This post was reviewed by Kira Gossack-Keenan, Brent Thoma and copyedited by Rebecca Dang.


