A 62 year old woman presents to your Emergency Department with a chief complaint of severe right eye pain. Upon further questioning, she reveals reduced vision in the affected eye and colored halos around lights. She reports a diffuse headache and two episodes of vomiting. A quick physical exam reveals significant conjunctival injection and a fixed, mid-dilated pupil. Does your differential contain acute angle closure glaucoma?
What is Acute Angle Closure Glaucoma?
Acute angle closure glaucoma is a condition characterized by raised intraocular pressure due to impaired outflow of aqueous humor from the posterior chamber of the eye.1 In a normal eye, the aqueous humor is produced in the posterior chamber by the ciliary process and proceeds to flow through the pupil to the anterior chamber and out through the trabecular meshwork into Schlemm’s canal.2 In acute angle closure glaucoma, the flow to the trabecular meshwork is blocked by contact between the lens and the iris resulting in accumulation of aqueous humor in the posterior chamber. This is referred to as “pupillary block.”3 As pressure in the posterior chamber rises, the iris is pushed further forward and causes the angle between the peripheral iris, trabecular meshwork, and cornea to close, hence the name acute angle closure glaucoma.
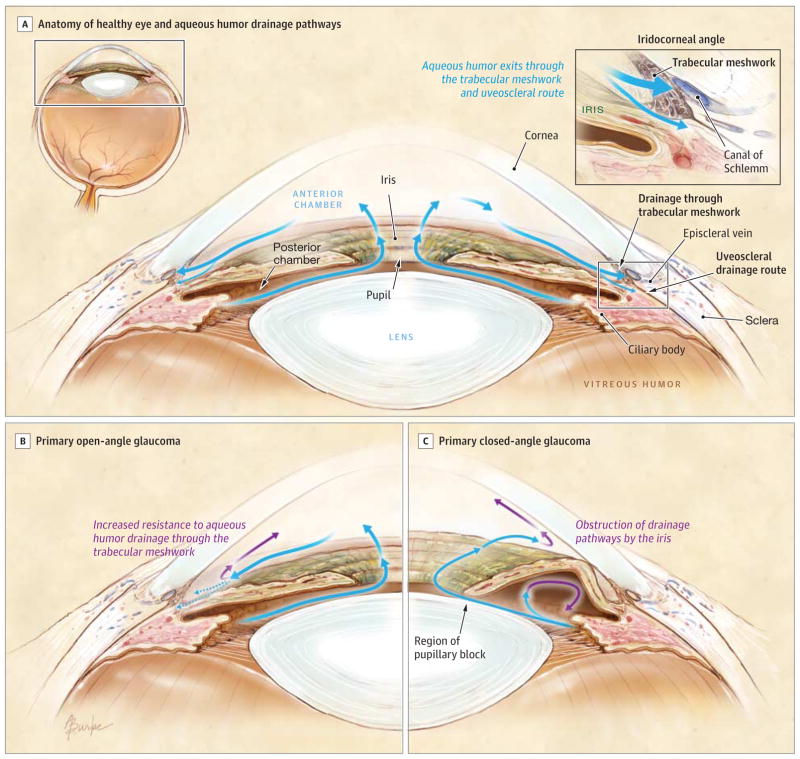
With permission from Weinreb RN, Aung T, Medeiros FA. The Pathophysiology and Treatment of Glaucoma. JAMA. 2014;311(18):1901-1911.
Clinical Presentation
As a result of the associated systemic symptoms, acute angle closure glaucoma carries a significant risk of being incorrectly diagnosed. A thorough history and physical examination with documented raised intraocular pressure is imperative for diagnosis.
History
Patients with acute angle closure glaucoma present with abrupt onset of pain in the affected eye. In addition patients may present with blurred vision, frontal headache, nausea and vomiting, photophobia, and colored halos around lights.4 Nausea and vomiting occurs as a result of autonomic stimulation, while blurred vision and colored haloes are a result of corneal edema.5 The onset is often precipitated by dilation of the pupil. When the pupil is mid-dilated, the contact between the iris and the lens is maximal and the iris thickens, which worsens pupillary block.6 Patients often describe onset of ocular pain when transitioning from a light to dark environment. Medications that dilate the pupil have the potential to precipitate an attack; these include adrenergic agents, drugs with anticholingeric effects, sulfa-based drugs, tricyclic antidepressants, anticonvulsants, and antiparkinsonian drugs.7 Other risk factors for acute angle closure glaucoma include a family history of the condition, being of Asian descent, female gender, hyperopic eyes, eyes with shallow anterior chambers, thick lens, and narrow irido-corneal angles. 6 As people age, their anterior chamber becomes more shallow and the lens thickens and moves anteriorly, 5 which makes this condition rare below the age of forty, with the peak incidence occurring in the sixth and seventh decades of life.8
Physical Examination
Patients with acute angle closure glaucoma present with conjunctival injection and a fixed, mid-dilated pupil measuring 5-6 mm in diameter.9 Due to the elevated intraocular pressure, the eye may feel hard to the touch. The visual acuity is often reduced. Raised intraocular pressure is necessary for the diagnosis of this condition, and is measured using a Tonopen. A pressure that exceeds 21 mmHg characterizes elevated intraocular pressure, however the pressure may exceed 60-80 mmHg. 2 Fundoscopic examination is often difficult due to the presence of corneal edema; however, one of the most notable features is a pale, cupped optic disc. Cupping occurs due to atrophy of the optic nerve fibers that results in the central cup of the optic nerve appearing enlarged. The normal cup-to-disc ratio is 0.3, but a patient with glaucoma will have a ratio of 0.6 or greater.10 Finally, a slit lamp examination is required for a complete eye examination in patients suspected of having acute angle closure glaucoma. Slit lamp examination will reveal a shallow anterior chamber and a cloudy cornea due to edema. 10 Gonioscopy, a procedure used to measure the depth of the anterior chamber, will be conducted by the Ophthalmologist.

Images from Glaucoma RCSI http://www.slideshare.net/hongchiong/glaucoma-rcsi
Treatment and Disposition
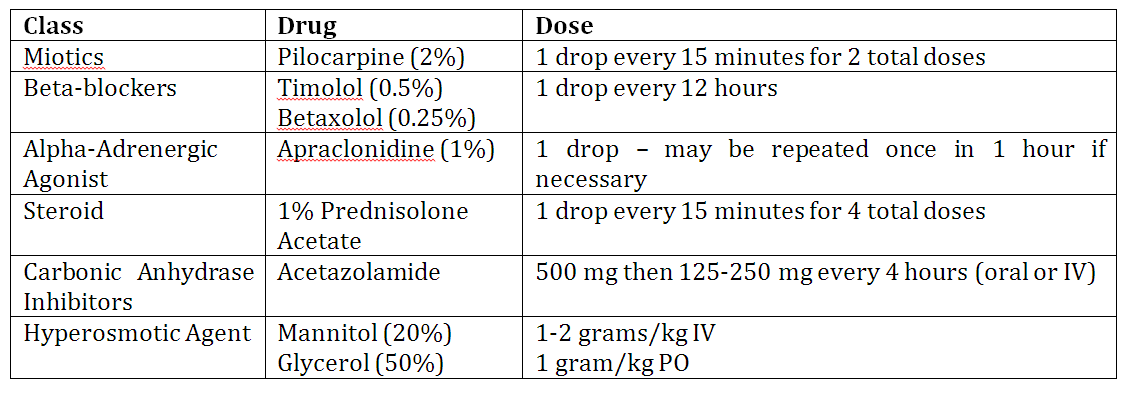
Prompt treatment is required for this condition, as the increased intraocular pressure can lead to optic nerve damage and vision loss. Immediate consultation with an Ophthalmologist is required. Pharmacological treatment options include:
Topical Agents
- Miotics – Pilocarpine is the miotic agent of choice. It acts on the muscarinic receptors of the iris, causing it to contract and leading to constriction of the pupil and the movement of the iris away from the angle. 5 This subsequently facilitates the opening of the trabecular meshwork and the outflow of aqueous humor. Be aware that pilocarpine may be ineffective at causing the iris to contract in the early stages of an acute attack because the elevated intraocular pressure can cause pressure-induced ischemic paralysis of the iris. 2 It is effective only once the pressure in the affected eye is reduced, leading to controversy regarding when pilocarpine should be administered. Some experts recommend initiating pilocarpine only after the intraocular pressure falls below 40 mmHg, while others recommend initiating it immediately upon diagnosis. Most experts recommend immediate administration to ensure availability once the ischemic paralysis has resolved.8
- Beta-blockers – These agents act by reducing the production of aqueous humor and reduce the intraocular pressure by 10-20%. 5 The same pulmonary and cardiac contraindications that apply to the use of oral beta-blockers also apply to topical beta-blockers, as they can cause systemic side effects including bronchoconstriction and bradycardia. As such, topical beta-blockers should be avoided in patients with asthma, chronic obstructive pulmonary disorder, and patients with second and third degree heart blocks.
- Alpha–Adrenergic Agonists – Topical Clonidine is the agent of choice. It lowers the intraocular pressure by reducing the production of aqueous humor and by increasing the aqueous outflow.
- Steroids – Prednisolone Acetate is the steroid most commonly used in the treatment of angle closure glaucoma. These agents help to reduce intraocular inflammation and in doing so, help to minimize the damage to the optic nerve.
Systemic Agents
- Carbonic Anhydrase Inhibitors – Acetazolamide is the typical agent used, and functions by reducing the production of aqueous humor through the inhibition of the enzyme carbonic anhydrase. These agents are contraindicated in patients with a sulfonamide allergy, sickle cell anemia, renal failure, and Addison’s disease. In addition, it is important to be aware that these agents may cause a metabolic acidosis, since this enzyme normally causes the dehydration of carbonic acid. As such, caution must be exercised in people with diabetes, liver disease, and COPD. 5
- Hyperosmotic Agents – Depending on the agent used, these can be administered either orally or intravenously. These agents decrease the intraocular pressure by causing an osmotic diuresis and reducing the vitreous volume. They should be used with caution in patients with cardiac, pulmonary, and renal issues.
Given the potentially vision compromising nature of this condition, all of these medical treatments should be initiated upon diagnosis. Intraocular pressure should be reassessed hourly to monitor response to treatment. While these pharmacological agents are often effective at lowering the intraocular pressure, patients will often require more definitive management with laser treatment, or occasionally, surgical treatment.
This post was copyedited by Rob Carey (@_RobCarey) and uploaded by Jesse Leontowicz (@jleontow)
References
Reviewing with the Staff
Acute angle closure glaucoma is an important cause of headache in the adult, and can be missed if not included in your differential diagnosis. While some of the physical examination findings are admittedly difficult to appreciate, measurement of the patient’s IOP with the Tonopen is simple and essential to making the diagnosis. The pharmacologic treatments listed in the article can temporize the patient, especially if you are in a centre without quick access to an Ophthalmologist, however their administration should not delay transfer and definitive management. Acute angle-closure glaucoma is an ophthalmological emergency, and the most important thing you can do is to recognize it and arrange for emergent definitive management.


