This episode of CRACKCast covers Rosen’s Chapter 149, Aspirin and Nonsteroidal agents. You will become well-versed in the presentation of Salicylism and how to manage it. The episode also touches on NSAID overdose, with rare severe complications.
Shownotes: PDF Here
[bg_faq_start]Key concepts
Salicylates:
- Salicylates are profoundly toxic and can be fatal.
- Salicylate overdose requires active assessment and treatment.
- The other NSAIDs generally have self-limited toxicity and respond to supportive measures only. There is no antidote for any of these drugs.
- Salicylism should be considered in the differential diagnosis of altered mental status in the elderly.
- Acidemia signifies loss of respiratory compensation and acceleration of toxicity.
- The Done nomogram should not be used in the evaluation and treatment of salicylate toxicity.
- Salicylate concentrations and blood gas draws should occur every 2 hours until salicylate level is less than 30 mg/dL (2.2 mmol/L) and is falling at least 10% between assays in the absence of measures to enhance elimination.
- Potassium stores are rapidly depleted in patients with salicylate intoxication and should be replaced with a goal serum level of 4.5 mEq/L.
- Mechanical ventilation should be avoided if possible in cases of severe salicylate poisoning. Acidosis may rapidly worsen due to loss of ventilation during the intubation procedure, and it is difficult to maintain ventilation at the level of physiological hyperventilation.
- If intubation is necessary, a bolus of sodium bicarbonate (50 to 100 mEq) is given before intubation and post-intubation minute ventilation is increased to match pre-intubation respiratory compensation.
- Enhanced elimination through urinary alkalinization with a sodium bicarbonate drip should be initiated in acute toxicity with a level >30 mg/dL (2.2 mmol/L).
- Consultation with nephrology and preparation for hemodialysis should occur if the salicylate concentration is 80 mg/dL (5.8 mmol/L) or is rising rapidly.
- Hemodialysis is recommended for signs of pulmonary or cerebral edema, coma, seizures, hepatic failure, renal failure, circulatory collapse, or refractory acidosis along with acute levels greater than 100 mg/dL (7 mmol/L) and chronic levels of 40 mg/dL (3 mmol/L).
- Altered mental status in the setting of salicylate toxicity warrants IV glucose supplementation.
NSAIDs:
- Most NSAID overdoses are asymptomatic or cause only minor symptoms.
- Ibuprofen, along with other propionic acid derivatives, has been associated with sporadic cases of aseptic meningitis.
- The management of NSAID overdose is supportive, and there is no specific antidote. Hemodialysis is reserved for patients with massive overdose and pH <7.1.
- Patients who have ingested a pyrazolone or fenamate require observation for possible seizures until 8 hours after ingestion.
Rosen’s In Perspective
Aspirin, or acetylsalicylic acid, is widely consumed for its analgesic, anti-inflammatory, and antiplatelet effects… however salicylate toxicity is not a benign condition and causes a complex set of life-threatening metabolic derangements with significant morbidity and mortality.
20-30 people die every year from ASA overdoses. The very young and very old are at particular risk!
It’s not just ASA that we worry about: other potential sources of salicylate toxicity include;
- Topical salicylates
- Oil of wintergreen
- Willow bark
- Bismuth subsalicylate
***Ingestion of oil of wintergreen is of particular concern given that 1 mL of 98% solution contains the equivalent salicylate of 1.4 grams of aspirin (this is one of those “one sip can kill” drugs).***
[bg_faq_end]Core questions
[bg_faq_start][1] What is the pathophysiology of ASA toxicity?
- Unpredictable GI absorption (2-4 hours, but can be longer – up to 12 hours)
- In the intestinal wall, liver, and red blood cells, aspirin is hydrolyzed to free salicylic acid, which reversibly binds to albumin
- Toxicity results primarily from salicylate interference with aerobic metabolism by uncoupling of mitochondrial oxidative phosphorylation. Inhibition of the Krebs cycle increases production of pyruvic acid and increases conversion to lactic acid.
- Metabolic rate increase → metabolic acidosis
- Tissue glycolysis → hypoglycemia and ketosis
- Only non-ionized particles can cross the lipophilic cell membrane and accumulate in the brain and other tissues. Because salicylic acid has a pKa of 3.5, the majority of salicylate is ionized and unable to enter tissue at the physiologic pH of 7.4. However, as serum pH decreases, more particles become un-ionized and cross the cell membrane and blood-brain barrier, markedly increasing the movement of salicylate into the tissues and central nervous system (CNS).
- This causes: seizures, coma, death, due to
- Cerebral edema
- Neuroglycopenia
- Direct nephrotoxicity – and renal failure
- Salicylate induced pulmonary edema – unknown mechanism
- Tinnitus: Cochlear toxicity is thought to be the result of alterations in N-methyl-D-aspartate (NMDA) activity, decreased blood flow, and increased membrane permeability
- This causes: seizures, coma, death, due to
[2] What is the toxic dose of ASA?
Mild – moderate toxicity = 150-300 mg/kg
Severe toxicity = > 300 mg/kg
Ingestions of >500 mg/kg are associated with death.
Fatal salicylate intoxication can occur after the ingestion of 10 to 30 g by adults and as little as 3 g by children.
Death typically results from severe central nervous system (CNS) toxicity with complete loss of function of the cardiorespiratory centers.
[3] How is Aspirin eliminated from the body?
ASA can reversibly bind to albumin, but free salicylate is eliminated by renal excretion.
At therapeutic salicylate concentrations, elimination follows first-order kinetics. Once serum salicylate concentrations are greater than 30 mg/dL (2.2 mmol/L), elimination follows zero-order kinetics. The metabolic pathways become saturated, and the pH-sensitive urinary excretion of salicylic acid determines the apparent half-life, prolonging significantly (up to 15 to 30hours) with large overdoses.
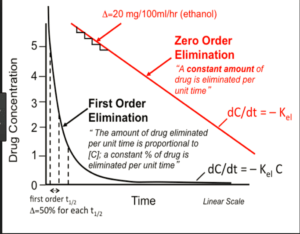
***Our goal urine pH is 7.5 – 8.0***
[4] Describe the clinical presentation of ASA toxicity. Describe the acid base disturbance and progression of toxicity in acute salicylate overdose.
Clinical presentation:
- Hyperventilation, N/V/dyspepsia (GI distress), tinnitus
- Severe hyperpnea, lethargy, hyperthermia (rare, late finding due to anaerobic metabolism)
- End organ dysfunction
- Coma, pulmonary/cerebral edema, seizures
- Renal failure
- Cardiovascular collapse
Acid base disturbances: any disturbance can occur!
- Respiratory alkalosis (unfortunately leads to bicarbonate losses)
- Metabolic acidosis (eventually high ASA levels depress the respiratory centre)
- With an anion gap
Refer to Box 144.1 in Rosen’s 9th edition for a handy table that describes the acid base disturbance and progression of toxicity in acute salicylate overdose.
- Early (0-4 hours; level 20-60 mg/dL):
- Respiratory alkalosis with alkalemia
- GI distress
- Mild-moderate hyperpnea
- Tinnitus
- Lethargy
- Moderate (2-12 hours; level 50-90 mg/dL):
- Respiratory alkalosis & metabolic acidosis with alkalemia or neutral pH
- Severe hyperpnea
- Lethargy or agitation
- Hyperthermia
- Severe (6-24 hours; level >80 mg/dL):
- Respiratory alkalosis or acidosis & metabolic acidosis with academia
- Severe hyperpnea
- Coma or acute delirium
- Hyperthermia
- Pulmonary or cerebral edema
- Seizures
- Cardiovascular collapse
[5] How is ASA toxicity managed?
KEY:
- Start urinary alkalinization
- Correct hypovolemia
- Urine output 2-3 mL/kg/hr
- Keep [K] >4.5 mM, correct any hypomagnesemia
- Give glucose for any CNS changes
In all cases of altered mental status, even in the face of a normal serum glucose measurement, supplemental intravenous (IV) glucose (0.5-1 g/kg) should be administered.
Refer to Box 144.2 in Rosen’s 9th edition for the detailed treatment of acute salicylate poisoning. A brief overview of the table is provided below.
- Treat dehydration
- Correct potassium depletion
- Consider activated charcoal (AC)
- Alkalinize urine
- Initiate hemodialysis
- Administer IV dextrose for any CNS abnormalities
Pearls & Pitfalls as per emDOCS
Pearls
- Optimize electrolyte imbalances prior to intubation, correct hypokalemia to >4 mEq/L
- If intubation necessary, give bicarb boluses (2 mEq/kg IV) and maintain vent settings at PRE-INTUBATION respiratory status, i.e. high minute ventilation and PCO2 < 20 mmHg. YOU CAN KILL A SALICYLATE TOXICITY PATIENT WITH INCORRECT VENTILATOR SETTINGS!
- Early hemodialysis
- Serial salicylate levels and VBGs q 2 hours
- Serum alkalinization more important than urine
- Avoid acidemia!
Pitfalls
- Forced diuresis with large volume IVF has no proven benefits, increased risk of pulmonary edema in patients already at risk for this
- Hyperventilation/tachypnea itself is not an indication for intubation à It is often a compensatory mechanism for the metabolic acidosis
- Overreliance on serum salicylate levels
- Not correcting hypokalemia
- Inadequate serum alkalinization (goal serum pH 7.45 to 7.55)
- Over-emphasizing urine alkalinization
- Failure to consider hemodialysis early in the clinical course
[6] When is dialysis indicated in patients with ASA toxicity (list at least 6)?
- Serum salicylate levels greater than
- 100 mg/dL (7.2 mmol/L) in acute
- 40 mg/dL (2.9 mmol/L) in chronic salicylate poisoning
- Altered mental status, including coma
- Seizure(s)
- Endotracheal intubation (other than for co-ingestions)
- Renal or hepatic failure
- Pulmonary edema
- Severe acid-base imbalance (pH <7.1-7.2)
- Rapidly rising serum salicylate level
- Failure to respond to more conservative treatment
[7] Which types of NSAIDS are more toxic?
- Pyrazolone (e.g., phenylbutazone) and fenamate (e.g., mefenamic acid) toxicity is associated with significantly higher morbidity.
- Phenylbutazone, a pyrazolone, is now rarely used (has been withdrawn from the market) because of its association with aplastic anemia and agranulocytosis. Although phenylbutazone overdose is rare, the course is much more severe than with other NSAIDs. Severely poisoned patients have early onset of GI distress, coma, seizure, hyperthermia, hyperventilation, alkalosis or acidosis, hypotension, electrocardiographic abnormalities, or cardiac arrest.
- Mefenamic acid, a fenamate, is associated with a relatively high incidence of seizures, which occur 2 to 7 hours after supratherapeutic ingestion. Rapid recovery is the rule with supportive care and IV benzodiazepines.
[8] How does NSAID toxicity manifest?
Most NSAID overdoses are asymptomatic or cause only minor symptoms.
Less common clinical effects include:
- Metabolic acidosis
- Muscle fasciculations
- Mydriasis
- Diaphoresis
- Hyperventilation
- Bradycardia
- Hypotension
- Dyspnea
- Tinnitus
- Rash
- Rare cases of coma, seizure, hypotension, and metabolic acidosis have been reported in massive overdose (400-500 mg/kg)
Renal dysfunction is seen only after large acute overdose and in association with a period of relative hypovolemia with hypotension. It is reversible and usually responds to supportive measures.
Ibuprofen is the most common NSAID ingested in overdose and most cases follow a benign, self-limited course. Symptomatic overdose occurs only after ingestion of at least 100 mg/kg, and symptoms develop within 4 hours of ingestion. Life-threatening toxicity is rare with most cases limited to mild GI disturbance that resolves in hours.
Treatment:
Decontamination: There is no evidence supporting the use of gastric emptying or AC in NSAID overdoses.
Enhanced Elimination: because of high protein binding and rapid metabolism, enhanced elimination is not useful in most cases. In the rare case of a massive overdose with pH <7.1, hemodialysis should be considered to correct acidemia. In this situation, hemodialysis may also remove the free drug once protein binding is overwhelmed. Plasmapheresis has been attempted in severe phenylbutazone poisoning.
Antidote Therapy: there is no specific antidote for NSAID poisoning. [bg_faq_end]
Wisecracks
[bg_faq_start]1) When are peak levels of ASA expected post-ingestion?
Salts of salicylic acid are rapidly absorbed intact from the gastrointestinal (GI) tract, with appreciable serum concentrations occurring within 30 minutes of ingestion of a therapeutic dose and peak levels in 2 to 4 hours. Large ingestions frequently delay gastric emptying. In addition, aspirin, particularly enteric-coated preparations, tend to form concretions in the stomach. These properties often result in prolonged absorption with rising serum levels for 12 hours or more. So make sure you draw serial levels – because ASA has a stuttering absorption profile for many hours (sometimes past 12 hours!)
Key point:
- Measure a salicylate concentration on arrival with a second sample obtained 2 hours later if the first level is detectable.
- If the second concentration is not substantially declining, obtain concentrations every 2 hours to monitor for continued absorption, which may be prolonged.
- Serum salicylate levels should be repeated every 2 hours until three consecutive levels are all <30 mg/dL and are decreasing by at least 10%-20% on each measurement while no longer undergoing therapy to enhance elimination.
2) What sequential acid-base abnormalities are expected? Why does each happen?
- Respiratory alkalosis – direct stimulation of the medullary respiratory center.
- Metabolic acidosis – multifactorial, loss of bicarb, ASA itself that is a weak acid, impaired Kreb cycle, impaired ability to excrete acids in urine, ketones, lactic acidosis
- “Acidemia” comes from impaired compensation mechanism: prolonged high serum concentrations eventually depress the respiratory center; bicarbonate loss in the urine, depletion of glucose and potassium
- Respiratory acidosis – from cerebral toxicity, neuroglycopenia, salicylate induced pulmonary edema
- Metabolic alkalosis from GI loss
a) How does this change in pediatrics?
Children can’t compensate as well as adults.
3) List 4 causes of induction of Hypokalemia in ASA toxicity.
- GI distress (vomiting) – stimulation of the medullary chemoreceptor trigger zone (CTZ)
- Respiratory alkalosis – hyperventilation (direct stimulation of respiratory centre)
- Increased renal excretion of Na, HCO3-, K
- Salicylate-induced increased permeability of the renal tubules with further loss of potassium
- Intracellular accumulation of sodium and water
PLUS: Inhibition of the active transport system, secondary to uncoupling of oxidative phosphorylation
4) What is the primary cause of mortality in ASA toxicity?
Primary cause of mortality = cerebral toxicity and metabolic acidosis
Mechanism: occurs through uncoupling of oxidative phosphorylation, and neuro/nephro/pulmonary toxicity.
***Note on the elderly patient:
Physiologic changes of aging predispose elderly patients to toxicity from chronic therapeutic ingestion. Decreased liver blood flow limits biotransformation of salicylate, and decreased renal function reduces salicylate clearance. Chronic ingestion decreases albumin binding, increasing the free salicylate that can enter the cell, and allows salicylates more time to pass through the blood brain barrier.
5) Describe mild, moderate and severe ASA toxicity
Mild/moderate: Salicylate toxicity initially generates GI distress followed by tachypnea, tinnitus, and hearing disturbance due to concentration-dependent reversible ototoxicity, diaphoresis, and an evolving anion gap acidosis.
Severe: as the toxicity progresses, hyperthermia, coagulopathy, cerebral and pulmonary edema, cardiovascular collapse, and ultimately, death, occur.
Chronic poisoning may be much subtler, manifesting as a waxing and waning combination of the above features of toxicity***
The mortality rate for chronic salicylate ingestion is 25 times greater than that of acute salicylate ingestion.
- <150 mg/kg: Minimal symptoms
- 150 – 300 mg/kg: Mild to moderate intoxication.
- >300 mg/kg: Severe intoxication. Metabolic acidosis, altered mental state and seizures
- >500 mg/kg: Potentially lethal
***Salicylism mimics sepsis, CNS infection, withdrawal syndromes, and alcoholic or diabetic ketoacidosis. This is especially true in chronic toxicity given that the serum salicylate concentration is relatively low.
6) How can ASA be effectively GI-decontaminated?
Activated charcoal (AC) has been shown to reduce salicylate absorption in both animal studies and human volunteer trials. Evidence in overdose is less clear.
Usually recommended as:
- Administering multiple-dose oral AC (25-50 g) every 2-4 hours if the patient’s GI, mental, and hemodynamic status tolerate, because large salicylate ingestions tend to form gastric concretions. AC is not used in chronic salicylate poisoning because the presentation occurs long after absorption.
7) List 4 priorities of ASA management and describe how each is carried out
- Reduce absorption with activated charcoal
- Urinary alkalinization
- IV
- 150 mEq of bicarb in D5W + 40 mEq of KCl @ 2-3 ml/kg/hr
- Goal urine pH = 8.0 and 2-3 ml/kg/hr urine output
- IV
- Glucose, potassium and magnesium repletion
- IV
- PO
- Consider advanced elimination
- Hemodialysis
Because salicylates have a low pKa and are renally excreted, alkaline urine traps the salicylate ion and increases excretion.
Salicylates are acidic compounds and therefore readily ionize in an alkali environment. Only in the non-ionized state can they traverse cell membranes. Thus, once ionized in the urine, they are effectively “trapped” and can be easily excreted.
8) Describe why intubating the ASA toxic patient is dangerous
Unless the patient is decompensating, early mechanical ventilation should be avoided.
“Similar to diabetic ketoacidosis, it is difficult to artificially achieve adequate minute ventilation that sufficiently matches the patient’s own respiratory compensation. In addition, the loss of ventilation during the intubation results in rapid loss of respiratory compensation and worsening acidemia. If the patient is sufficiently critically ill to require intubation, worsening of acidosis during apnea is potentially harmful. Thus, we recommend administration of 50-100 mEq (1-2 amps) of sodium bicarbonate (NaHCO3) immediately prior to the procedure irrespective of the serum pH. Attempt to adjust the tidal volume post-intubation to match the pre-intubation PCO2 level. In addition, establish an elevated minute volume and obtain frequent blood gases to guide ventilator management to maintain respiratory compensation.” – Rosen’s
Podcast Summary
The Poison Review summed it up best with the following:
- Salicylate-poisoned patients are almost universally volume depleted at the time of presentation” and volume resuscitation is an early priority.
- Salicylate levels should be checked frequently (many toxicologists recommended doing this every 2 hours) until they are clearly decreasing and the patient is improving clinically.
- Since salicylate levels are reported different ways, pay close attention to the units. [100 mg/dL = 1000 mg/L = 7.24 mmol/L]
- A “normal” anion gap does not rule-out salicylate toxicity.
- Since salicylate toxicity increases CNS utilization of glucose, serum glucose levels may not reflect CNS levels.
- If intubation is required, the clinician should pay careful attention to maintaining hyperventilation and avoiding worsening academia.
- Early nephrology consultation is crucial.
This post was uploaded and copyedited by Samuel Hogman


