You’re ready to start a quality improvement (QI) project aimed at improving the effectiveness of the communication surrounding patient prognosis by providers. Your Plan-Do-Study-Act (PDSA) cycles involve retrospective chart reviews, surveys with patients to understand their experiences, and 1:1 key informant interviews with inter-disciplinary staff members in your department. You’ve learned about navigating ethics in quality improvement , and have reached out to your institution’s Research Ethics Board or QI leadership to determine the process for ethical approval/waiver. You start preparing your application but what about informed consent? Is it appropriate to propose that consent is not needed and ask for a waiver? Are there components of your QI project that do require consent? When is consent needed and how do you ask for it?
Welcome to this HiQuiPs post that takes a deeper dive into requirements and best practices around consent! And don’t forget to clarify best practices in your local jurisdiction and organization early on, as they may differ from our proposed framework here.
Consent in QI: How does it work?
Since you know your project on prognosis communication is QI and not research, it does not fall under the purview of national research governance policies (e.g., Canada’s Tri-Council Policy Statement (TCPS2) Article 2.5; 2018), and does not need to meet their requirements for obtaining consent. Even so, QI projects often involve accessing Personal Health Information (PHI) outside the circle of care (e.g., your chart reviews!), so it’s important to understand the laws in your local jurisdiction around consent. In Ontario, Canada, consent requirements around PHI are governed by the Personal Health Information Protection Act (PHIPA, 2004). Under PHIPA, QI projects that are internal to your organization may use PHI previously collected for clinical purposes without obtaining additional consent. QI projects usually include measures that rely on aggregate or process data, for example the average communication effectiveness score as dropped anonymously by patients in a survey collection box or the monthly number of anonymized patient complaints pertaining to staff communication, making obtaining individual consent less relevant and impracticable. So for the retrospective chart review component of your project, it is indeed appropriate to request that consent not be obtained from patients (e.g., a waiver)! To make sure to protect participants, project leads and the organization, your organization’s review process will likely require you to explain how you will meet de-identification standards (e.g., removing names and direct identifiers) and keep the information protected (e.g., securely stored on internal password-protected computers).
Consent for QI Surveys, Interviews & Focus Groups
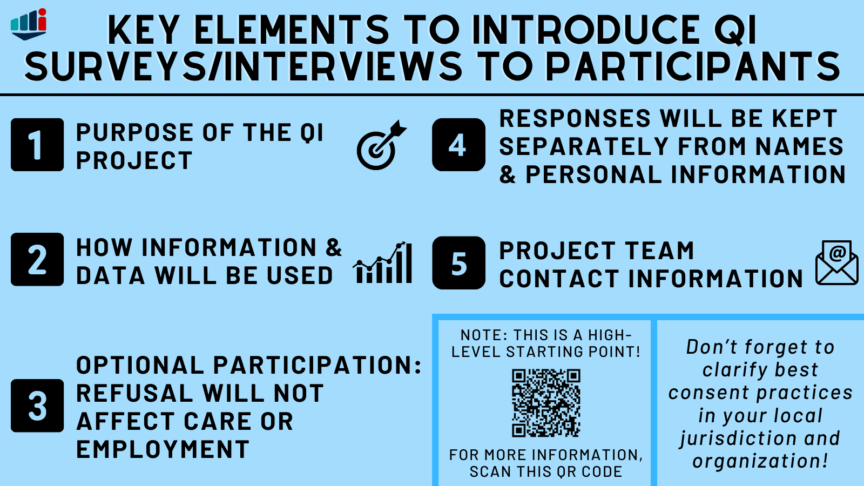
When data is collected specifically for QI surveys, interviews, or focus groups however, participants should be provided with information on the project so they can make an informed consent decision before taking part! Establishing this as a best practice will help to ensure that QI work is firmly rooted in sound ethical principles. So returning to your project, you should compose a brief introductory script that will accompany your survey and interview guide to inform potential participants on your project and help them decide whether they’d like to participate. So what information do you need to include in this script? Key components include a statement that the project is QI, a description of the project’s purpose, that participation is optional, how the information will be used, who will have access to the data, approaches to de-identification, and where data will be stored. A contact email for the project team and your organization’s ethical review body (whether REB or QI review, as locally relevant) should be included in case participants have questions. As a helpful starting point, the University Health Network (UHN)’s Quality Improvement Review Committee (QIRC) has developed this QI Introductory Script Template.
Consent in the Digital Age
While we’ve tackled more practical elements of consent for a traditional QI project here so far, consent is a complex and often controversial paradigm, especially in 2021! Parallel to rapid progressions in technological discovery and innovation in recent years, QI methodologies have also advanced to include the use of apps, smart phones, artificial intelligence, social media, and other complex digital tools. While offering brilliant promise for systems improvements, these methods can introduce complex challenges like systematic bias, tracking whereabouts, re-identification, and commercial uses of data. Technology and QI methods are advancing at the speed of light but even with amendments in recent years, laws and policies around consent tend to move at a glacial pace. Indeed, PHIPA was created in 2004 and so was Facebook! Resting on traditional QI consent approaches (e.g., consent waivers) may create an unintended chasm of risk for organizations and participants. Inspired work is needed to further modernize consent practices, and reconcile these vastly different paces of change!
Conclusion
So there you have it! You’ve designed a robust QI project, and are now informed on best consent practices for each component. You’ve requested a waiver of consent from your institution for your retrospective chart reviews, and you’ve prepared introductory scripts to accompany your surveys and interview guides that honour the principles of informed consent. Good luck on your project!
Senior editor for this post was Dr. Lucas Chartier (@chartierlucas)
This post was copyedited by Maryam Zadeh (@_MaryamZadeh)