This episode of CRACKCast covers Rosen’s Chapter 82, Pericardial & Myocardial Disease. This episode covers two diseases that can be challenging both in diagnosis and management and how to approach them in the ED.
Shownotes – PDF Here
[bg_faq_start]Rosen’s in Perspective
- These are challenging diseases — both in managing and diagnosing! They can present with a multitude of symptoms….
- Anything that causes pericarditis can lead to → pericardial effusion → tamponade / constrictive pericarditis
- It’s scary that the incidence of this disease in the ER is UNKNOWN…
- Remember our anatomy for the pericardium:
- There are parietal and visceral layers – that potential space
- The parietal layer is attached to the diaphragm, sternum and the vertebral column.
- Blood supply from internal mammary artery and innervation from the phrenic nerve
- Normally 15-35 mls volume
- Pericardial effusion – occurs when the lymphatic or venous drainage of the heart is obstructed.
- There are parietal and visceral layers – that potential space
1) List eight causes of pericarditis.

- Idiopathic!
- A specific cause is found in LESS than 20% of patients!!
- Treatment:
- NSAIDs for 2 weeks
- Ibuprofen 600 mg q6hrs x 1 week; if not effective switch to colchicine or indomethacin
- Infectious
- Lots of weird and wonderful infections
- Bacterial and viral co-infections can exist
- g. varicella-zoster and superinfection of Staph. Aureus
- Post-trauma
- Post MI, cardiac surgery, thoracic sx, trauma – penetrating injury
- Usually appears 4-12 days post
- Metabolic
- Systemic autoimmune diseases
- Tumours
- Aortic dissection
- Post MI, cardiac surgery, thoracic sx, trauma – penetrating injury
- NSAIDs for 2 weeks
2) Describe typical pain of pericarditis & expected labwork abnormalities
- Chest pain
- Sharp
- Pleuritic
- Varies with position
- Relieved by sitting forward and worse lying down/deep breath in/swallowing
- May radiate to shoulders/diaphragm
- Hx of fevers and myalgia
- The friction rub – is typically only heard in sound-proofed cardiologists offices
***there is no single test that is diagnostic for pericarditis***
[bg_faq_end][bg_faq_start]3) What is the typical sequence of ECG changes in patients with pericarditis? (the three stages)
- Given the difficulty of making the diagnosis of pericarditis, the ECG is our most reliable tool.
- ESR and WBC are neither sensitive nor specific
- Troponin elevation suggests – Myocarditis, myopericarditis, or MI
There are three stages:
- First hours to days of illness
a) Diffuse ST seg. Elevation
i) Reciprocal depression in aVR and V1
ii) ***unlike MI, pericarditis has concave upward ST segments, and no T wave inversions, no dynamic changes, no reciprocal changes, and no evolution of Q waves***
b) PR seg depression - Normalization of ST segments
- . Flattening of the T waves → T wave inversion
- ECG normalizes (occasionally T waves can stay inverted)
Bottom line: ACS can be difficult to distinguish from acute pericarditis

4) Describe the treatment of pericarditis associated with: Uremia, Neoplasm, and SLE
Uremia
- Due to renal failure or dialysis
- Pericarditis can occur with acute renal failure or with chronic renal failure
- Look for the effusion!
- Trxt:
- Find any underlying cause (infection)
- Intensive dialysis
- **NSAIDS are contraindicated! And ineffective.
- Steroids for those who don’t respond to dialysis
Neoplasm
- Think lung, breast, lymphoma, leukemia metastatic disease (primary dz very rare)
- At risk for malignant pericardial effusions – causing death due to tamponade
- New symptoms: SOB, cough, palpitations weakness, dizzy, hiccups, fatigue
- Pericardiocentesis
- With sclerosing or chemo agents
SLE / RA / connective tissue diseases
- Are all at risk for constrictive pericarditis or tamponade
- Trxt:
- Corticosteroids
5) Outline the management of Dressler’s syndrome.
- 20% of pts experience a “different quality of chest pain” 2-4 weeks post MI
- +/- low grade fever and rub
- ECG changes are often masked by the ACS ECG changes
- At risk for dysrhythmias and CHF
- Mgmt:
- 1-3 days of ASA 325 mg daily
HOWEVER…..
- Dressler’s syndrome is the LATE post-MI pericarditis
- Can also occur post PE and post-surgery
- NO anticoagulants – because of risk of hemorrhage
- Ibuprofen or Indomethacin
6) What is the pathophysiology of cardiac tamponade? Describe the mechanism of hypotension in pericardial tamponade and list 4 expected findings on physical examination.
- Beck’s Triad:
- JVD; hypotension, muffled heart sounds
- Look for electrical alternans or pulsus paradoxus
- Remember: Commonly seen in CANCER, TRAUMA and UREMIA
- Comes down to nature of fluid, rate of accumulation and state of cardiac function
- Acute collection problem, 50-200ml can cause tamponade physiology.
- Chronic can compensate, can drain LITRES!
***Stages: 1) Accumulation in parietal pericardium – 2) Fluid accumulating faster than the rate of the parietal pericardium ability to stretch – 3) Accumulation that exceeds the body’s ability to increase blood volume to support right ventricle filling pressure.
Net result is increased pericardial pressure leading to decrease preload/ventricle compliance/filling
REMEMBER: The most important factor is the RATE of accumulation
Need 200-250 mL to show cardiomegaly on CXR

7) Describe the procedural steps in pericardiocentesis
Check out:
http://www.emcurious.com/blog-1/2014/11/28/the-pericardiocentesis
https://emin5.com/2016/07/11/pericardiocentesis/
http://www.sonoguide.com/pericardiocentesis.html
https://lifeinthefastlane.com/ccc/pericardiocentesis/
Blind technique (LITFL):
- Subxiphoid approach
- Long 18-22 G needle attached to syringe
- Insertion: between xiphisternum and left costal margin
- Direct towards the left shoulder at 40 degree angle to skin
- Continual aspiration as needle approaches RV
- Once pericardial fluid aspirated, can insert cannula into pericardial space
- Attach a 3 way tap and remove fluid with improvement in haemodynamics
U/S Guided
Subxiphoid / Parasternal / Modified Apical approach
Similar as above, but add realtime U/S. Cardiac probe.
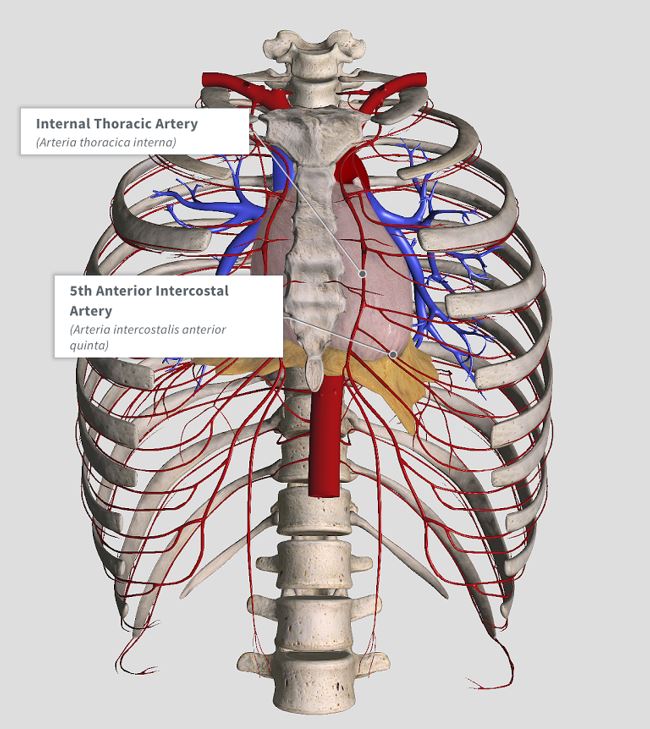
Watch out for the LAD, Internal Thoracic, Mammary and Intercostal vessels!!!
8) List 4 causes of pneumopericardium and one specific PEX finding
- Malignant: Esophageal cancer / lung cancer erosion
- Iatrogenic: Post-EGD/thoracic surgery
- Infectious: necrotising staph. Aureus
- Post-traumatic: blunt chest trauma
Hamman’s crunch!!
[bg_faq_end][bg_faq_start]9) List five causes of constrictive pericarditis.
From Uptodate:
- Idiopathic or viral – 42 to 61 percent
● Post-cardiac surgery – 11 to 37 percent
● Post-radiation therapy – 2 to 31 percent, primarily after Hodgkin disease or breast cancer
● Connective tissue disorder – 3 to 7 percent
● Post-infectious (tuberculous or purulent pericarditis) – 3 to 15 percent
● Miscellaneous causes (malignancy, trauma, drug-induced, asbestosis, sarcoidosis, uremic pericarditis) – 1 to 10 percent
[bg_faq_end][bg_faq_start]
10) What is the pathophysiology of purulent pericarditis? List 5 organisms responsible for infectious pericarditis? How is it managed?
- Direct spread from an intrathoracic focus of infection, including extension from a myocardial focus or direct contamination from trauma or thoracic surgery
- Hematogenous spread
- Extension from a subdiaphragmatic suppurative focus
- Aureus
- Pneumo
- Salmonella
- Candida
- Histoplasma infection (Ohio and Mississippi Valley)
Drain it! Abx. Everyone needs surgery…
— From Uptodate
[bg_faq_end][bg_faq_start]11) Describe the pathophysiology of hypertrophic cardiomyopathy
- Prevalence – 1:500
- Autosomal Dominant
- Hundreds of different mutations are known
- Its a genetic disease of sarcomere proteins – causing sarcomere disarray and whorling / scarring
- Leading to a hypertrophied LV (in the absence of another cause for the LV to be hypertrophied)
- This thickening is usually asymmetrical – more the septum than the free wall
- But the hyperT can be anywhere..
- The LV and RV cavities are usually normal
- All this is thought to stem from abnormal cardiac protein construction – in response to physiologic stress, the heart tries to adapt by building a big cellular structure – hyperT
- The thickness of the LV and degree of outflow tract obstruction correlate with disease severity.
- This also leads to impaired ventricular filling
12) Describe the clinical exam and ECG findings associated with HCM
- Average age of dx – 30-40 yrs!!
- Sx:
- Dyspnea, chest pain, syncope, near-syncope, palpitations
- Px:
- Low S4 gallop
- Harsh midsystolic murmur
- Worse with valsalva or changing from standing to squatting position (change in preload and afterload – its a dynamic murmur)
- Bifid arterial pulse
ECG: Abnormal in 90%
- PACs and PVCs
- Multifocal ventricular ectopy
- Ventricular and supraventr. Dysrhythmias
- LVH
- ST segment changes
- T wave inversions
- LAE
- Abnormal / dagger Q waves
- Diminished or absent R waves in the lateral leads
13) List 5 RFs for sudden death in HCM
- Family member with a history of sudden cardiac death
- History of syncope / SVT’s
- Massive LVH
- Abnormal hypertensive response to exercise
- Young age of diagnosis
- Stimulant abuse
- Ischemic heart disease
14) A patient with known hypertrophic cardiomyopathy presents to the ED with acute cardiogenic pulmonary edema causing mild hypoxia. What is the general approach to management in the ED? Explain your choices.
Most people with HCM are on long-term betablocker therapy. Some may be on CCB’s.
While we would typically think about using nitro in CHF….this is the WRONG answer…
Nitro decreases ventricular volume – a bad thing in HCM.
So our treatment of choice:
- MOVIEs
- Put the defib pads on!
- Increase preload (leg lift / fluid challenge)
- Call cardio
- If in cardiovascular collapse:
- IV phenylephrine
- IV propranolol or esmolol
- Beta-blocker
- Amiodarone or sotalol for dysrhythmias (AF or VT)
- ICD
To recap: NO vasodilators in HCM. They drop the peripheral vascular resistance and increase the LV outflow tract obstruction and filling pressures = leading to worsening heart failure and hypotension!
Afib in the HCM patient is treated in a similar way as the general population – cardioversion, rate control and anticoagulation.
[bg_faq_end][bg_faq_start]15) List four causes of dilated cardiomyopathy (DCM)
- Genetic mutations of the cytoskeleton / sarcomere proteins
- Environment…
- Myocarditis
- Peripartum DCM
- ETOH (toxins)
- Ischemia
- Autoimmune – e.g. SLE
16) Describe ECG findings of dilated cardiomyopathy
DCM is defined by abnormal ventricular contractility with an EF <45%
- Nonspecific :
- Poor R wave progression
- Intraventricular conduction delay
- LBBB
- Holter showing
- PVCs or occasional VT
17) List 5 RFs for developing a dilated cardiomyopathy
- Primary cardiac muscle disease – e.g. hx of myocarditis
- Infectious
- Post-ischemia
- Auto-immune disease
- ETOH abuse
- Cocaine abuse
18) In what time frame would one expect peripartum DCM?
- Last 3 months of pregnancy or first 5 months postpartum
19) List 5 causes of restrictive cardiomyopathy
- Tropical endomyocardial fibrosis
- Amyloidosis
- Scarcoidosis
- Hemochromatosis*** only treatable kind!
- Slcerderma
- Neoplasm
- Glyocogen storage diseses
20) List 8 common pathogens responsible for myocarditis, and 3 non-infectious causes of myocarditis
This is a tough disease to diagnose
Think about it in people with vague symptoms:
- Flulike symptoms, fever, fatigue, myalgias, vomiting/diarrhea, dyspnea, chest pain
- Signs:
- Fever, tachycardia, tachypnea, dysrhythmias,
- ***Unfortunately no symptom or sign is sensitive or specific to the diagnosis***
- Cardiac auscultation is usually normal
- In Kids: look for grunting respirations and intercostal retractions +/- wheezing
- Harbingers of badness:
- Cyanosis, toxic looking, ventricular dysrhythmias
- ECG:
- Sinus tach, wide QRS, low electrical activity, AV block, long QT interval
- Labs:
- Troponin – of some use, but its elevation time scale is unknown
- WBC and ESR are of no diagnostic value
- IgM viral titres may show a specific type of virus
- Dx:
- ECHO u/s may show hypokinesis
- MRI is the test of choice, and may have similar sensitivity to biopsies
- Harbingers of badness:
Bottom line: Think of this diagnosis!
- Especially in the otherwise healthy patient who presents with an ECG that looks like an ACUTE MI, elevated biomarkers, CHF or dysrhythmias

Infectious
- Advenovirus
- Parvovirus 19
- herpes virus 6
- CMV
- Toxoplasma gondii
- Chagas disease (#1 cause in south america)
- Coxsackie B virus
- Influenza
Non-infectious
- Drug related (ETOH, chemo drugs)
- Allergic reactions (post antibiotics)
- Chemicals (arsenic)
21) Describe the stages of viral myocarditis and the management at each stage
- Supportive management is key
- Acute : The necrosis from the virus invading the myocytes
- Antivirals – pleconaril or ribavirin
- Sub-acute : host cellular immune response causing cytotoxicity = autoimmune injury
- Immunosuppression (steroids haven’t shown much benefit)
- IVIG
- Chronic: the toxic chemical effects produced by the pathogen = fibrosis, cardiac dysfunction and dilated cardiomyopathy
- CHF treatment
- VAD
- Cardiac transplant
Wisecracks
[bg_faq_end][bg_faq_start]1) What are some functions of the pericardium?
- Keeps the heart in position
- Lubricates the heart’s surface
- Prevents the spread of infection
- Prevents cardiac overdilation
- Augments atrial filling
- Maintains normal pressure-volume relationships of the cardiac chambers….
2) What are Chagas Disease and Trichinosis? List bizz-buzz features for each.
Chagas:
- Protozoal infection in Central America – Trypanosoma cruzi
- Very cardiotoxic if someone develops symptoms from the disease
- Think about it in anyone traveling from Latin America with myocardial dysfunction
- Treatment is two antiparasitic drugs for > 60 days
Trichinosis:
- Ingestion of cysts found in undercooked meat (e.g. pork) – Trichinella spiralis
- But now found in hunters, immigrants who raise their own pork, international travelers
- Systemic flu-like illness, biopsy or serologic studies needed
- Can cause myocardial involvement
- Treatment: Corticosteroids and anti-helminthic drugs
3) What are the expected cardiac findings in Lyme disease and how is it treated?
Causes a reversible AV blockade leading to heart block. Antibiotics can usually reverse this blockade!
[bg_faq_end][bg_faq_start]4) How does sarcoid affect the heart?
Sarcoid leads to granuloma formation – location determines the effects.
In the septum = conduction defects
In the pap muscles = mitral regurg
In the ventricular walls = wall motion abnormalities
These mechanical problems are usually unresponsive to standard therapies…..its one of those weird situations where you may prescribe systemic corticosteroids for VT!
[bg_faq_end][bg_faq_start]5) Amyloidosis?
Massive amyloid deposition – increased cardiac weight and restrictive cardiomyopathy – leading to CHF. Standard therapies are usually ineffective, leading to death in a year.
[bg_faq_end]This post was uploaded and copyedited by Colin Sedgwick (@colin_sedgwick)


