This episode of CRACKCast covers Rosen’s Chapter 124, Acid Base Disorders. This chapter covers a simple approach to acid base disorders and ABG interpretation, including the differential diagnosis for the identified disorders & treatment options.
Shownotes – PDF Here
[bg_faq_start]Key Point
Patients with an acute severe metabolic acidosis rely on a robust respiratory compensation; in these cases, the adequacy of the ventilatory response should be assessed and augmented, with non-invasive or invasive ventilation, if needed.
- The strong ion difference = ([Na+ + K+ ] − [Cl− ]). When significantly less than 40, an acidosis is present.
- The delta gap (ΔG) = (AG − 12) − (24 − [HCO3 − ]). Its calculation determines if the anion gap is accounted for by the change in serum bicarbonate concentration. An elevated anion gap and ΔG more than 6 indicates that a metabolic alkalosis in addition to a metabolic acidosis is likely to be present.
- Patients who have a chronic respiratory acidosis (eg, in chronic obstructive pulmonary disease) are at risk for dangerous alkalemia if they are ventilated with routine parameters. Blood gas analysis in these cases should be performed frequently and settings titrated to the serum pH.
- Alcoholic ketoacidosis may be manifested similarly to diabetic ketoacidosis but is much less common; insulin is contraindicated in alcoholic ketoacidosis.
- When an elevated anion gap is recognized, the initial assessment focuses on identifying one of four causes: ketoacidosis, toxic ingestions, lactic acidosis, and renal failure. Typically, only chronic renal failure causes significant acidosis.
- Anion gap = Na+ − (Cl− + HCO3 − ). Causes of an elevated anion gap include ketoacidosis, lactic acidosis, toxins metabolized to acids, and renal failure.
- When the cause of an elevated anion gap is determined to be lactate or ketones, diagnostic efforts are directed at identifying the cause of the lactic acidosis or ketoacidosis.
- Sodium bicarbonate is not recommended for the empirical treatment of acidemia; it is an option in cases of severely depressed pH thought to pose an immediate life threat.
Rosen’s In Perspective
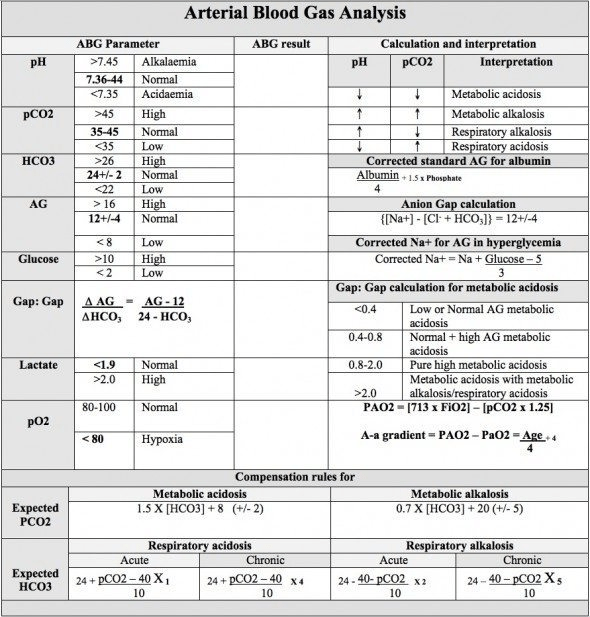
Many basic cellular processes are sensitive to small changes in serum pH; the kidneys, lungs, and physiologic buffers determine serum pH, which is normally between 7.36 and 7.44. Serum pH is determined by the relative concentrations of bicarbonate (HCO3) and carbon dioxide (Paco2) = [1.5 × serum HCO3 − ] + [8 ± 2]; when two of these variables are known, the third may be calculated. Most blood gas analyzers measure pH and Paco2 and report a calculated [HCO3 − ].
When only a primary disturbance and its corresponding compensation are present, it is described as a simple acid-base disorder. A mixed acid-base disorder exists when more than one primary disturbance occurs simultaneously.
Key thought process: Is this a simple ABD or a mixed ABD?
Often, acid-base disturbances are first identified when the results of laboratory tests ordered to evaluate the patient’s symptoms demonstrate alterations in the bicarbonate level, pH, or Paco2. The possibility of an acid-base disorder is suggested by clinical events such as toxic ingestions, severe vomiting, or diarrhea, as well as in patients with diseases primarily affecting the lungs and kidneys. All critically ill patients and all patients being mechanically ventilated should have an assessment of their acid-base status. When an acid-base disturbance is identified or suspected, elucidation of its underlying cause(s) is central to appropriate management.
Painful arterial blood sampling is unnecessary for the evaluation of acid-base disturbances. Paco2, HCO3−, and pH values taken from peripheral venous, central venous, intraosseous, and capillary blood are all suitable for acid-base assessment.
[1] Describe an approach to acid-base problems
Simple acid-base disorders are categorized by the serum pH, Paco2, and HCO3 − concentrations (See Table 116.1 for changes to pH, PaCO2, HCO3– and expected compensation).
When the primary disturbance is identified, the next step is to determine its cause and whether an appropriate compensation has occurred. An inappropriate compensation suggests that the process underlying the primary disturbance has hindered an appropriate response or that more than one primary disturbance is present.
If you’re serious about getting better at this, just go check out: https://lifeinthefastlane.com/investigations/acid-base/
There are a bunch of very helpful worksheets on this site, including the 1-2-3-4-5 rule for determining if there has been an adequate metabolic compensation for resp. acidosis/alkalosis.
But here’s the approach from LITFL: (and a worksheet from them below)
Interpret the ABGs in a stepwise manner:
1. Determine the adequacy of oxygenation (PaO2)
- Normal range: 80–100 mmHg (10.6–13.3 kPa)
2. Determine pH status
- Normal pH range: 7.35–7.45 (H+ 35–45 nmol/L)
- pH <7.35: Acidosis is an abnormal process that increases the serum hydrogen ion concentration, lowers the pH and results in acidaemia.
- pH >7.45: Alkalosis is an abnormal process that decreases the hydrogen ion concentration and results in alkalaemia.
3. Determine the respiratory component (PaCO2)
4. Primary respiratory acidosis (hypoventilation) if pH <7.35 and HCO3– normal.
- Normal range: PaCO2 35–45 mmHg (4.7–6.0 kPa)
- PaCO2 >45 mmHg (> 6.0 kPa): Respiratory compensation for metabolic alkalosis if pH >7.45 and HCO3– (increased).
- PaCO2 <35 mmHg (4.7 kPa): Primary respiratory alkalosis (hyperventilation) if pH >7.45 and HCO3– normal. Respiratory compensation for metabolic acidosis if pH <7.35 and HCO3– (decreased).
5. Determine the metabolic component (HCO3–)
- Normal HCO3– range 22–26 mmol/L
- HCO3 <22 mmol/L: Primary metabolic acidosis if pH <7.35. Renal compensation for respiratory alkalosis if pH >7.45.
- HCO3 >26 mmol/L: Primary metabolic alkalosis if pH >7.45. Renal compensation for respiratory acidosis if pH <7.35.
*Whether an appropriate respiratory compensation to a metabolic acidosis has occurred can be approximated by comparing the Paco2 to the last two digits of the serum pH.
In a primary metabolic acidosis with appropriate respiratory compensation, these two values should be similar; that is, a patient with a serum pH of 7.20 should have a Paco2 of 20 mm Hg.
If the serum pH is 7.17 and the Paco2 is 30 mm Hg, the respiratory compensation is less than expected and a primary respiratory acidosis is present. This is commonly seen in patients whose dominant condition causes a metabolic acidosis but also impairs respiration, such as sepsis.
“THE DELTA GAP”
The delta gap (ΔG) describes the difference between the deviation of the anion gap (AG) from normal and the deviation of the serum bicarbonate concentration from normal:
![]()
Conceptually, calculation of the delta gap tried to determine whether the anion gap is accounted for by the change in serum bicarbonate concentration. In patients with an elevated anion gap and a delta gap greater than +6, meaning that the serum bicarbonate level is significantly higher than would be predicted by the number of unmeasured anions, a metabolic alkalosis in addition to a metabolic acidosis is likely to be present.
This is commonly seen when the dominant acidosis condition causes severe vomiting (eg, diabetic ketoacidosis). In patients with an elevated anion gap and delta gap more negative than −6, meaning that the serum bicarbonate level is significantly lower than expected given the anion gap, a normal anion gap metabolic acidosis is likely be present in addition to the elevated anion gap metabolic acidosis. This is usually seen when a lactic acidosis complicates severe diarrhea.
[2] List a DDx for RespAcidosis, RespAlkalosis, MetAcidosis, MetAlkalosis
RespAcidosis
Respiratory acidosis occurs when hypoventilation leads to an inappropriately elevated Paco2 and resulting acidemia. Any condition that reduces minute ventilation may cause respiratory acidosis.
It is the ACUTE resp. acidosis that kills! Restore oxygenation and ventilation STAT while you find the cause!
A way of thinking through the DDx:
Won’t breathe
Can’t breathe (weak muscles / CNS lesion)
Can’t breathe enough (narrowing of the airways / air trapping )
It is thought that spontaneous respiration cannot sustainably reduce Paco2 beyond 10 to 12 mm Hg, which is the appropriate compensatory respiratory alkalosis for a metabolic acidosis that drives the pH down to about 7.10.
Here’s the list from Rosen’s (Box 116.1)
Causes of Respiratory Acidosis
Acute
- Airway obstruction
- Pulmonary disease (pneumonia, asthma, pulmonary edema, aspiration pneumonitis)
- CNS depression (recreational drugs, intracranial catastrophe, neuromuscular disorders, thoracic trauma)
Chronic
- Lung disease (COPD, IPF)
- Neuromuscular disorders (ALS, muscular dystrophy, obesity hypoventilation)
After 3-5 days…an appropriate metabolic compensation for respiratory acidosis is an increase of approximately 3.5 mEq/L in the serum HCO3− concentration for every increase of 10 mm Hg in Paco2. A patient with chronic respiratory disease resulting in a baseline Paco2 of 60 mm Hg would therefore be expected to have a serum bicarbonate level of approximately 30 mEq/L and a slight acidemia, because without another primary acid-base abnormality, correction is never to a normal pH.
Respiratory Alkalosis
- Increased minute ventilation = low PaCO2 and increased pH
- The dx of exclusion is anxiety-related hyperventilation.
- Here’s a ddx: (From box 116.2)
- Hyperventilation caused by hypoxia
- High altitude
- Severe anemia
- Ventilation-perfusion mismatch
- Central hyperventilation
- Voluntary or psychogenic
- Cerebrovascular accident
- Increased intracranial pressure (tumor, hemorrhage, trauma)
- Toxic or pharmacologic
- Salicylates**
- Caffeine, nicotine
- Catecholamines
- Thyroxine
- Pulmonary
- Pulmonary embolism
- Pneumonia
- Pulmonary edema
- Asthma
- Mechanical hyperventilation (iatrogenic)
- Endocrine
- Pregnancy
- Paco2 between 31 and 35 mm Hg, serum pH between 7.46 and 7.50, and serum bicarbonate concentration between 18 and 22 mEq/L. Thus, eucapnia (Paco2 ≅ 40 mm Hg) may represent hypoventilation in pregnant patients.
- Hyperthyroidism
- Pregnancy
- Septicemia
- Hepatic encephalopathy
- Hyponatremia
- Hyperventilation caused by hypoxia
*Suicidal aspirin ingestions often produce the classic syndrome of tinnitus, hyper-
Thermia (morbid finding), confusion, and a variety of metabolic derangements, ultimately leading to seizures, coma, and cardiovascular collapse. Chronic salicylism, however, is notoriously subtle in its presentation and occurs in older sicker patients who seemingly have more likely explanations for their symptoms. Salicylate toxicity should be strongly considered in these patients, as well as those with an unexplained respiratory alkalosis.
Metabolic Acidosis
- Reduced serum bicarbonate level due to:
- Intrinsic acids
- Extrinsic acids
- Impaired acid excretion
- We divide this broad category into Elevated AG MA and Normal AG MA
See questions 3-4
Metabolic Alkalosis
Metabolic alkalosis occurs from loss of H+ or retention of HCO3− It is usually a consequence of prolonged vomiting or nasogastric suction or a compensation for chronic respiratory acidosis. The differential diagnosis includes a variety of predominantly endocrine and electrolyte disorders
Causes of Metabolic Alkalosis (Box 116.5):
Volume-contracted (saline-responsive)
- Vomiting, gastric suction
- Diuretics (contraction alkalosis)
- Postrespiratory acidosis
- Ion-deficient baby formula
- Chloride-secreting colonic villous adenomas
Euvolemic, Volume-Expanded (saline-resistant)
- Hyperaldosteronism
- Hypercortisolemia
- Severe potassium depletion
- Adenocarcinoma
- Bartter’arterrcin
Unclassified
- Hypoalbuniemia
- Milk-alkali syndrome
- Penicillin and related compounds
- Hypercalcemia or malignancy
- Massive transfusion (from citrate, especially with renal deficiency)
Big ones:
- Vomiting
- Hyperaldosteronism
- Hypercalcemia of malignancy
[3] List causes of an elevated AGMA
Common unmeasured anions that cause an elevated anion gap include:
- Lactate,
- Keto acids (usually from diabetic ketoacidosis),
- Acidic products of exogenous toxins (eg, the toxic alcohols),
- Organic acids that accumulate in renal failure
Aka this is known as “KULT”
According to Rosen’s: The causes of elevated anion gap metabolic acidosis are classically remembered by the mnemonic MUDPILES; however, it is more useful to focus on the identity of the unmeasured anion (Box 116.3).
For example, if the cause of an elevated anion gap metabolic acidosis is found to be lactate or ketones, the next step is to determine the cause of lactatemia or ketonemia.
Here’s the list:
Causes of Elevated Anion Gap Metabolic Acidosis – From Rosen’s.
KETOACIDOSIS (3)
Diabetic ketoacidosis
Alcoholic ketoacidosis*
Starvation ketoacidosis
*Patients with AKA may demonstrate a high anion gap, but a mixed acid-base disorder may be present due to concomitant ethanol withdrawal, which may cause a respiratory alkalosis and metabolic alkalosis from vomiting. As a result, the serum pH can be acidemic, normal, or Alkalemic. A urine dipstick can detect acetoacetate but not β-hydroxybutyrate; this causes an apparent worsening ketonuria as the patient metabolizes β-hydroxybutyrate to acetoacetate, although the patient is actually recovering. The treatment of AKA is dextrose-containing fluids; insulin is contraindicated.
LACTIC ACIDOSIS (3) = global ischemia, cellular poison (oxidative phosph. stopped), impaired lactate clearance)
Global tissue ischemia
- Shock
- Grand mal seizure (in status unresponsive to standard treatment,
- consider isoniazid toxicity)
Focal end-organ ischemia (limb ischemia, mesenteric ischemia)
Inadequate blood oxygenation
- Hypoxia (from airway or breathing disorder)
- Carbon monoxide, methemoglobinemia
Inability of tissues to use oxygen from cellular poisons
- Cyanide (or nitroprusside therapy), hydrogen sulfide, aspirin, iron
Impaired lactate clearance
- Liver failure
- Metformin or phenformin (increased risk with concomitant renal
- insufficiency [creatinine > 1.5 mg/dL]) congestive heart failure,
- coexisting metabolic acidosis, and exposure to intravenous
- radiologic contrast media)
- Reverse transcriptase inhibitors (for HIV infection therapy)
RENAL FAILURE (the body’s naturally production of acids = sulfate, phosphate, urate, hippurate)
TOXINS METABOLIZED TO ACID
Toxic alcohols (methanol, ethylene glycol)*
* As the parent compounds are converted to their toxic metabolites, the osmol gap closes, and the anion gap widens.
Toluene
Paraldehyde
Aspirin
SEVERE RHABDOMYOLYSIS
Serum albumin accounts for most of the physiologic (normal) anion gap, usually between 9 and 15.
For example, a chronically malnourished alcoholic may present with alcoholic ketoacidosis but may also have longstanding hypoalbuminemia, which causes a substantial increase in the serum bicarbonate concentration. An acute ketonemia will reduce the serum bicarbonate level to normal, resulting in no anion gap.
In addition to albumin, classic unmeasured anions such as lactate and ketones can also be measured and incorporated into a diagnostic pathway. For example, a lactate level of 4 mEq/L can be presumed to account for an anion gap of 16 mEq/L, but not an anion gap of 25 mEq/L; in this case, other unmeasured anions should be sought. Unmeasured cations are also present and may account for an elevated anion gap in a case of hypomagnesemia or hypocalcemia, often in combination with hypokalemia.
[4] List causes of NAGMA
This is much less life threatening than elevated AGMA!
Most cases encountered in the emergency department (ED) are caused by gastric loss of bicarbonate in the setting of diarrhea.
Causes of Normal Anion Gap Metabolic Acidosis (Box 116.4)
GI HCO3– Loss
- Diarrhea
- Colostomy or ileostomy
- Enteric fistulas
- Ion exchange resins (ex. Kayexalate)
Renal HCO3– Loss
- Renal tubular acidosis
- Tubulointerstitial renal disease
- Hyperparathyroidism
Rapid normal saline infusion
- Urologic procedures
- Ureterosigmoidostomy
- Ureteroileal conduit
Ingestions
- Acetazolamide
- Calcium chloride
- Magnesium sulfate
Other
- Hypoaldosteronism
- Hyperkalemia
- Toluene
NOTE:
Overall, a normal anion gap does not exclude clinically significant acidosis or high concentrations of unmeasured anions. Direct measurement of compounds that often produce an elevated anion gap (eg, lactate, ketones, toxic alcohols) should still be pursued when there is clinical concern, despite a normal anion gap.
[5] List 5 complications of bicarbonate therapy
This is a list in the text, so it’s good to know!
- Paradoxical CNS acidosis—because sodium bicarbonate does not readily cross the blood-brain barrier, serum alkalinizes much faster than the cerebrospinal fluid. As the serum pH rises, minute ventilation slows and carbon dioxide, which readily crosses the blood-brain barrier, delivers an additional acid load to the CNS.
- Hypokalemia, which can cause respiratory muscle weakness, impairing respiratory compensation, hypocalcemia, hypernatremia
- Volume overload
- Hyperosmolality
- Overshoot alkalosis
So what to do??….administration of sodium bicarbonate is unlikely to be beneficial in most cases of acidemia caused by endogenous acid production (eg, lactic acidosis, ketoacidosis), sodium bicarbonate therapy is theoretically more attractive for conditions in which the underlying problem is bicarbonate loss, which generally causes a hyperchloremic or normal anion gap metabolic acidosis, or impairment of acid secretion, such as renal failure. Many of the acidosis syndromes caused by exogenous toxins (eg, aspirin, tricyclic antidepressants) call for bicarbonate as a specific therapy; these cases are distinct from the empirical use of bicarbonate to treat the acidemia itself. Bolus dosing of hypertonic (8.4%) sodium bicarbonate is ineffective and should not be used to treat hyperkalemia.
Hyperkalemic patients (especially if acidemic) may be treated with an isotonic sodium bicarbonate infusion, as described above. Acidemia causes hyperkalemia as H+ is brought into cells in exchange for K+.
So, as long as you’re not just “treating” the acidosis with bicarb (i.e. you know the cause and are addressing it as well) bicarb may be a reasonable choice for the conditions mentioned above (Tox, renal loss, renal failure).
NOT to be used empirically in cardiac arrest, NOT to be used in undifferentiated acidemia or alkalemia from lactic/ketoacidosis.
Although it is commonly recommended to treat a serum pH less than 7.1 with sodium bicarbonate (1 mEq/kg),17 we believe that best evidence currently supports withholding bicarbonate therapy as an empirical treatment for acidemia in favor of treating its underlying cause.
An iso-tonic bicarbonate drip is prepared by adding 150 mEq sodium bicarbonate (three crash cart ampules, which are usually 50 mL of 8.4% solution) to 1 L of 5% D5W, infusing as slowly as the clinical situation permits (eg, 75–200 mL/hr). Sodium bicarbonate should generally not be added to normal saline because of concern of hypertonicity.
Winter’s equation (Paco2 = [1.5 × serum HCO3] + [8 ± 2]) can be used to calculate the expected arterial Pco2 for the serum bicarbonate level. If the Pco2 is higher than expected, the patient may be fatiguing and require ventilator support.
Wisecracks
[1] BONUS: What are causes a LOW anion gap?
Elevated concentrations of unmeasured cations may cause a low anion gap (<3 mEq/L), such as in cases of:
- Lithium toxicity
- Hypergammaglobulinemia seen in multiple myeloma.
- Bromide toxicity may cause spurious hyperchloremia, and hypertriglyceridemia may cause spurious hyponatremia, which can also cause a low or negative anion gap.
Other causes: think about what else the anion gap usually includes to be in the 6-12 range: unmeasured anions such as albumin, sulfate, phosphate, and citrate. So, isolated depressions of these may cause a low anion gap.
Hypoalbuminemia would be such a case.
[2] BONUS: Why do patients with hyperventilation have lip and extremity paresthesias, carpal pedal spasm, muscle cramps, lightheadedness, and syncope?
Blood pH alters the binding affinity of calcium for albumin. When the pH lowers, calcium loses some affinity for albumin (increasing free calcium); when the pH increases, calcium binds more strongly to albumin (decreasing free calcium). Thus, clinical features of respiratory alkalosis include those from hypocalcemia—lip and extremity paresthesias, carpal pedal spasm, muscle cramps, lightheadedness, and syncope.
The classic paper bag technique to cause rebreathing – works through the placebo effect, rather than changing PaCO2 – and has risks associated with it.
[bg_faq_end]This post was uploaded and copyedited by Colin Sedgwick (@colin_sedgwick)


