All the content from the Blood & Clots series can be found here.
CanMEDS Roles addressed: Medical Expert, Collaborator
Case Description
You are assessing a 55-year-old male patient in the emergency room who is presenting with headache, fever, and neck stiffness. He needs a lumbar puncture to rule out bacterial meningitis. However, he is on apixaban 5 mg twice daily for atrial fibrillation with a CHADS65 score of 1 point for hypertension. His body mass index and kidney function are within normal limits. When would it be safe to perform this procedure?
Main Text
Neuraxial procedures are classified as high bleeding risk interventions, mainly because of the potentially severe consequences of a bleeding event, such as spinal hematoma with subsequent paraplegia in the worst case, and less so based on the absolute number of bleeding associated with the intervention1. As such, patients are required to be off anticoagulants before undergoing this procedure.
When is it safe to perform neuraxial procedures in patients on direct oral anticoagulants (DOACs)?
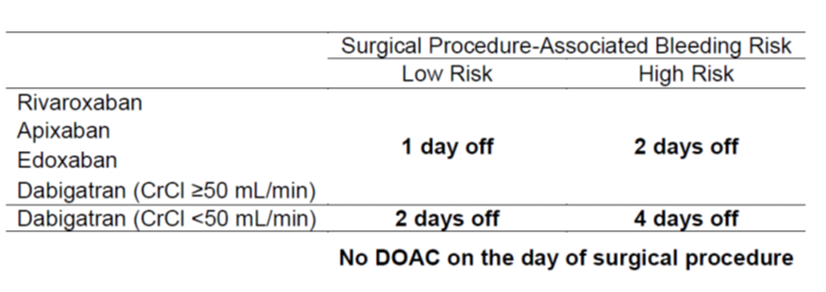
The Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) cohort study represents the best available evidence to answer this question. It was designed to evaluate the safety of a perioperative anticoagulation management strategy in patients on DOACs for atrial fibrillation who undergo elective surgery 2. Based on DOAC pharmacokinetic properties, a standardized protocol was established which required patients undergoing a high bleeding risk procedure, such as any neuraxial anesthesia, to hold apixaban, rivaroxaban or dabigatran the 2 days prior to the procedure (corresponding to approximately 5 DOAC half-lives). An additional 48 hours were required for patients on dabigatran with an estimated glomerular filtration rate (eGFR) below 50 mL/min. DOACs were resumed 2-3 days after procedure. Using this approach, the 30-day postoperative rate of major bleeding was below 2% for any DOAC, which is considered to be safe. Even though, the PAUSE study was not powered to evaluate the safety of the perioperative DOAC management in patients undergoing surgery with neuraxial anesthesia, its results in combination with pharmacologic sense re-assured our practice following the PAUSE protocol in those patients. Furthermore, we feel comfortable extrapolating the results from elective surgery to urgent or emergent procedures if the DOAC clearance is not compromised and there is no concomitant antiplatelet use.
Table 1. Preoperative DOAC interruption schedule as per PAUSE study 2.
Current guideline recommendations from the American Society of Regional Anesthesia and Pain Medicine (ASRA) are more conservative with regards to direct inhibitors of factor Xa, recommending that rivaroxaban, apixaban or edoxaban be discontinued 72 hours prior to neuraxial anesthesia. For dabigatran, the ASRA recommends an approach based on kidney function: 72 hours prior if eGFR is 80 mL/min or greater, 96 hours if the eGFR is between 50 and 79 mL/min, and 120 hours if the eGFR is between 30 and 49 mL/min. The ASRA guidelines were published prior to the PAUSE study and are based on expert opinion considering DOAC pharmacokinetic properties and case reports.
Do coagulation tests help in patients on DOACs?
While performing coagulation tests, it is important to note that INR/PTT are not to be used to evaluate DOAC anticoagulation. The use of agent-specific anti-factor-Xa levels for rivaroxaban, apixaban and edoxaban or dilute thrombin time for dabigatran, may be appealing to determine anticoagulant activity in patients on DOACs; however, no reference ranges for a normal level exist for these tests. Furthermore, the recent PAUSE trial showed that a strategy based on DOAC pharmacokinetic is safe for perioperative anticoagulation management. DOAC levels were measured just before the procedure in 832 of 1007 high-bleeding-risk patients but results were not available to treating physicians and did not influence patient management. Ten out of 832 patients had an anti-factor Xa level or dilute thrombin time above 50 ng/mL and 61 patients an anti-factor Xa level or dilute thrombin time of 30 to 49.9 ng/mL 2. In the entire cohort with DOAC level measurement (n=2541, 84.5%), 158 (6.2%) patients had DOAC levels above 50 ng/mL and 363 (14.3%) patients a level between 30 to 49 ng/mL. In those patients, the risk of bleeding was not increased compared to patients with normal DOAC levels 3. Our opinion is that coagulation tests are best reserved for situations in which an urgent procedure is required and the last dose of DOAC is unknown or the case is complicated by severe renal impairment. When an urgent intervention associated with a high bleeding risk is required, the International Society on Thrombosis and Haemostasis considers an anti-factor Xa level or dilute thrombin time below 30 ng/mL as safe to proceed with the intervention when drug levels are likely to fall over time based on the time the last DOAC dose was taken 4.
Neuraxial procedures in patients on warfarin
The ASRA and European guidelines differ regarding recommendations for neuraxial anesthesia in patients on warfarin 5 6 7. For daily practice, the most relevant difference is the INR value deemed to represent normalized coagulation. While the ASRA recommends an INR of ≤1.2 for neuraxial anesthesia in patients on chronic warfarin, the ESA and the British guidelines considers an INR ≤1.4 as sufficiently low. The ASRA recommendation is based on a study including 23 patients who stopped warfarin 5 days prior to measure clotting factors. Twenty-one patients had an INR of 1.2, 1 patient an INR of 1.3 and 1 patient an INR of 1.4. While median activities of factor II, VII, IX, and X were normal in the patients with an INR of 1.2, this was not the case in the 2 patients with higher INRs. Of note, not all patients with an INR of 1.2 had normal clotting-factor concentrations. Regardless of cut-off used, the administration of reversal agents such as vitamin K and/or prothrombin complex concentrates should be based on an individual risk-benefit approach as discussed in a previous article.
The anesthesiologist performing the lumbar puncture informs you that their national guidelines specify a different duration between discontinuation of direct oral anticoagulants and performance of neuraxial procedures. How will you navigate this situation?
It is worthwhile discussing the results of the recent PAUSE study which support theoretical models based on pharmacokinetics and is the best clinical data available. Discussing the case based on apixaban half-lives may be appealing to anesthesiologists who are used to apply pharmacokinetic knowledge in their daily practice. Ultimately, the decision whether it is safe to perform a procedure or not is made by the person performing the intervention.
Case Conclusion
The patient took the last dose of apixaban on the morning before presentation to the emergency room (24 hours). His medications were reviewed and no concomitant antiplatelet therapy or drug interfering with DOAC clearance identified. An apixaban-specific anti-factor Xa level was performed confirming the presence of apixaban (anti-Xa level 90 ng/mL). In this situation, a lumbar puncture is contraindicated, and presumptive treatment of the meningitis required. The apixaban should be held for an additional 24 hours. The intervention can then be performed without repeating of the coagulation test.
Main Messages
- A pharmacokinetic-based approach to interruption of DOAC intake for neuraxial procedures appears to be safe.
- Coagulation tests are best reserved for situations in which the timing of last intake of DOAC is unclear or if an urgent procedure is required, or in case the patient is on warfarin.
References
1. Spyropoulos AC, Brohi K, Caprini J, et al. Scientific and Standardization Committee Communication: Guidance document on the periprocedural management of patients on chronic oral anticoagulant therapy: Recommendations for standardized reporting of procedural/surgical bleed risk and patient‐specific thromboembolic risk. J Thromb Haemost. August 2019:1966-1972. doi:10.1111/jth.14598
2. Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern Med. November 2019:1469. doi:10.1001/jamainternmed.2019.2431
3. Douketis JD, Spyropoulos AC, Duncan J, et al. Effect of DOAC Type and DOAC Dose, and Residual DOAC Level on Risk for Perioperative Major Bleeding and Arterial Thromboembolism. International Society on Thrombosis and Haemostasis. . Published July 9, 2019.
4. Levy JH, Ageno W, Chan NC, et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. February 2016:623-627. doi:10.1111/jth.13227
5. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional Anesthesia in the Patient Receiving Antithrombotic or Thrombolytic Therapy. Regional Anesthesia and Pain Medicine. April 2018:263-309. doi:10.1097/aap.0000000000000763
6. Gogarten W, Vandermeulen E, Van Aken H, Kozek S, Llau JV, Samama CM. Regional anaesthesia and antithrombotic agents: recommendations of the European Society of Anaesthesiology. European Journal of Anaesthesiology. December 2010:999-1015. doi:10.1097/eja.0b013e32833f6f6f
7. Harrop-Griffiths W, Cook T, et al. Regional anaesthesia and patients with abnormalities of coagulation. Anaesthesia. August 2013:966-972. doi:10.1111/anae.12359
All the content from the Blood & Clots series can be found here.
This post was reviewed by Teresa Chan, Anson Dinh and copyedited by Rebecca Dang.



